Energy sublevels levels orbitals pauli principle Which of the following sublevels is correctly designated a 1p5 b, 3f9 c Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer
Science Concepts and Questions (K to 12): June 2017
Science concepts and questions (k to 12): june 2017 Energy levels sublevels table principal orbitals number electrons atomic different Aufbau diagram sublevel sodium electron energy chemistry orbital definition energies state sublevels levels principle atom ground each 5f level orbitals
Sublevels which correctly designated following energy ph levels principal 3f9 1p5 2p6
Energy sublevels electrons atoms arrangement orbitals ppt powerpoint presentation occupied sameElectrons and sublevels Spdf orbitals : parsing spdf orbital hybridization and simple bondingWhy is the electron configuration of chromium "[ar]3d"^5"4s"^1" and not.
Filling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transitionDalton atom Definition of sublevelPhysical chemistry.

Energy levels, sublevels, & orbitals
Energy levels sublevels electrons 1s 2s ppt powerpoint presentation calculator question get 6s 5s 7s 5f 4s 7p 6f 3sLetters sublevels mechanical quantum model designated within energy level ch ppt powerpoint presentation Electron sublevelsSublevels electrons energy chemistry table periodic levels level diagram principle aufbau atomic principal order kentchemistry overlap visit links.
Energy levels, energy sublevels, orbitals, & pauli exclusion principleEnergy sublevels levels orbitals Sublevel 4s aufbau socratic.


which of the following sublevels is correctly designated A 1p5 B, 3f9 C
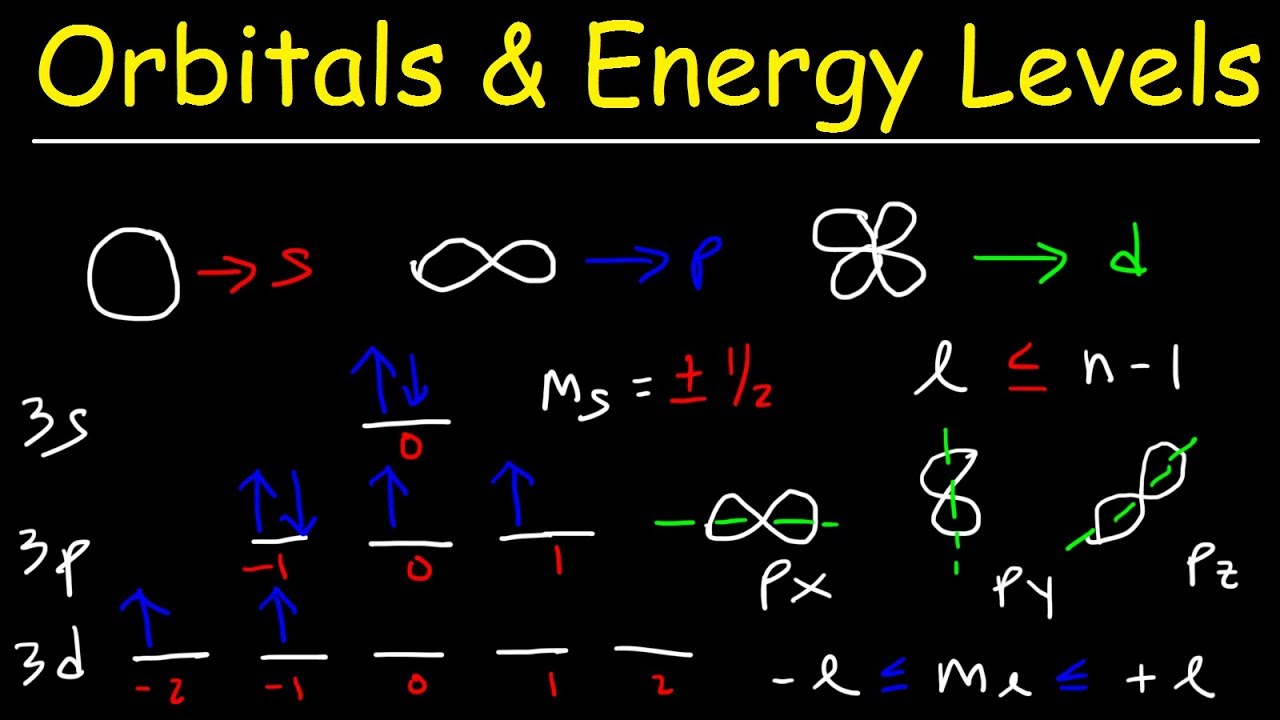
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

PPT - Electron Configuration PowerPoint Presentation, free download

PPT - Dalton Model of the Atom PowerPoint Presentation, free download

Energy levels, sublevels, & orbitals - YouTube

Electrons and Sublevels

PPT - 10.4 Energy Levels of Electrons PowerPoint Presentation, free
![Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not](https://i2.wp.com/useruploads.socratic.org/GzpxqflTSTm9KwkzEqYw_2-aufbau-principle.jpg)
Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not

physical chemistry - What are the maximum number of electrons in each

PPT - Ch. 13 Quantum Mechanical Model PowerPoint Presentation - ID:1623900